Powerful mechanical moment.
Golovna beauty and health The electron imparts a powerful mechanical torque to the pulse L s, called spin.
Spin is the invisible power of the electron, similar to its charge and mass.
The spin of the electron is confirmed by the magnetic moment P s, proportional to L s and the straightening of the protracted side: P s = g s L s, g s - Hydromagnetic relation of spin moments.
Projection of the magnetic moment of direct vector B: P sB =eh/2m= B , deh=h/2, B =Bohr magneton.
The underlying magnetic moment of the atom p = the vector sum of magnetic moments included in the electron atom: P a = p m + p ms.
Dosvid Stern and Gerlach. .
The Zeeman effect is called the splitting of energy levels after the influx into atoms of a magnetic field.
The splitting of the lines leads to the splitting of the spectral lines into a number of components.
The splitting of spectral lines when a magnetic field is applied to a magnetic field is also called the Zeeman effect. Zeeman splitting is explained by the fact that an atom possessing a magnetic moment j induces additional energy in the magnetic field E = - jB B, jB is the projection of the magnetic moment onto the direct field. jB =- B gm j , E = B gm j , ( j =0, 1,…, J). The energetic rhubarb splits into sub-levels, and the amount of splitting lies in the quantum numbers L, S, J of that level. Power mechanical and magnetic moments (spin) PRIMED BACK
. The Schrödinger equation allows one to explore the energy spectrum of water and folded atoms. The experimental measurement of the equal energies of atoms showed that there is no obvious escape from the theory.
The exact vimirs revealed the fine structure of the ranks.
All the trees, except the main one, are split into a number of very close trees.
The spin of the electron was suggested by the research of Stern and Gerlach (1922) to prevent the splitting of a narrow beam of atoms under the influence of a non-uniform magnetic field (in a homogeneous field the moment does not change the orientation atsion; the bodies in the heterogeneous field of veins are gradually collapsing either along the field or against the laying. directly in front of the field).
The unwound atoms of the planet are in a spherically symmetrical state, with an orbital momentum equal to zero. The magnetic moment of the system, with the orbital thrust of the electron (as in the classical theory), is directly proportional to the mechanical moment. If the remaining value is equal to zero, then the magnetic moment must also be equal to zero. Therefore, the external magnetic field is not to blame for flowing into the structure of atoms in the main body. Evidence shows that such an influx is. Before the end of the day, there was a splitting of a beam of atoms from the sliver, meadow metals
and water, ale first of all Just beware two bundles
, however, on the opposite side and spread symmetrically to the beam without a magnetic field. It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. The results of further investigations are brought to the conclusion, That the splitting in the magnetic field of a beam of atoms of the first group of the Periodic System, which obviously occurs near the s-site, into two components is explained by two possible states of the spin magnetic moment of the valence electron. The magnitude of the projection of the magnetic moment onto the direct magnetic field (itself means the effect of dilation), found from the studies of Stern and Gerlach, was comparable to the so-called Bohr magneton- orthohelium), which indicate three possible projections on the direction of the magnetic field of the orbital streams of the total spin of two electrons (+h, 0, -h).
Thus, the lack of facts has made it necessary to attribute a new internal level of freedom to electrons.
For a complete description, I will describe the order of the three coordinates, or be another three quantities, so that to establish a quantum-mechanical set, it is necessary to specify the magnitude of the spin projection on the forward direction (the spin modulus is not necessary to indicate, as evidence shows Every part of the veins does not change not under any circumstances). Bohr magneton The spin projection, as well as the projection of the orbital momentum, can change by a multiple of . The fragments were careful about two orientations of the electron spin. Uhlenbeck and Goudsmit assumed that the projection of the electron spin S . The fragments were careful about two orientations of the electron spin. Uhlenbeck and Goudsmit assumed that the projection of the electron spin z.
Honestly, we can take two meanings: = ±h/2 U 1928 r. Dirac has removed the relativistic quantum equation for the electron, from which the spin of the electron is absorbed
h/2
without any special hypotheses. This is how an electron has a spin of 1/2 and produces a proton and neutron.
The spin of the photon is equal to 1. If the fragments of the photon mass are equal to zero, then two or even three projections of +1 and -1 are possible.
These two projections in Maxwell's electrodynamics indicate two possible circular polarizations of the electromagnetic coil and opposite the directional arrow, which are directly wider.
THE POWER OF CONTINUOUS MOMENTUM IMPULSE.
I orbital moment M, and spin moment S are quantities that take on more than quantum discrete values. Let us now look at the final moment of the impulse, which is the vector sum of the predicted moments. The operator of constant momentum is significant in terms of the sum of operators and Operators do not interact, since the operator is coordinates, but the operator is not coordinates. jB =- B gm j , E = B gm j , ( j =0, 1,…, J). Can you show what Then the projections of the total angular momentum do not commute one with the other in the same way as the projections of the orbital angular momentum. The operator interacts with any projection, as evidenced by the traces that the operator and the operator with any (or one) projection correspond to physical quantities that lie up to the same moment. . The fragments were careful about two orientations of the electron spin. Uhlenbeck and Goudsmit assumed that the projection of the electron spin The operator commutes in the same way as operators. It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. ; The position of the electron near the central force field was designated by three quantum numbers: It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. n, l, m. The position of the electron near the central force field was designated by three quantum numbers: It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. Then the Hvilov’s function with the urahuvannya’s back is to blame.
For skin thermal Operators do not interact, since the operator is coordinates, but the operator is not coordinates. n,l mi maemo (2 l+ 1) positions that vary by the orientation of the orbital moment (number The position of the electron near the central force field was designated by three quantum numbers:), the skin of which is split into two parts, which are divided by the back. l In this manner, obviously 2(2
+ 1) - multiple virogen. n,l If we now take into account the weak interaction of the spin with the magnetic field of the orbital streams, then the energy will become deposited even in the spin orientation before the orbital moment.
The change in energy for such interactions is little equal to the difference in energy between levels. And the new lines are close to each other. Thus, the difference in the orientation of the spin moment in relation to the internal magnetic field of the atom can explain the similarity of the multiplicity of spectral lines. The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin. This conclusion is confirmed by experimental data.
We now move up to the numbering of the ranks of the atom with the arrangement of the multiplet structure. The fragments were careful about two orientations of the electron spin. Uhlenbeck and Goudsmit assumed that the projection of the electron spin In terms of the spin-orbital interaction, neither the orbital momentum nor the spin has no significant significance with the singing energy (operators and interact with the operator). The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin. The fragments were careful about two orientations of the electron spin. Uhlenbeck and Goudsmit assumed that the projection of the electron spin The operator commutes in the same way as operators. And the new lines are close to each other. According to classical mechanics, the precession of vectors is small and near the vector of constant torque, as shown in Fig. And the new lines are close to each other. 20. Every moment is deprived of your life. l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. (l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. A similar situation occurs in quantum mechanics. And the new lines are close to each other. When the spin interaction occurs, only the last moment has a different value from the given energy (the operator interacts with the operator). l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. Therefore, the regulation of spin-orbital interaction should be classified according to the values of the constant moment. The position of the electron near the central force field was designated by three quantum numbers: And the new lines are close to each other. = The last moment is quantized according to the same rules as the orbital moment. It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. (The position of the electron near the central force field was designated by three quantum numbers: It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. How to enter the quantum number l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. j The position of the electron near the central force field was designated by three quantum numbers:, which sets the moment
And the new lines are close to each other. = 1/2, 3/2, 5/2, … ; The position of the electron near the central force field was designated by three quantum numbers: And the new lines are close to each other. J And the new lines are close to each other..

, That And the new lines are close to each other. = l+ Ѕ і And the new lines are close to each other. = |l And the projection onto a straight line is 0 May have meaning, when
= l +
= S), since the spin is parallel to the orbital moment, i l= | And the new lines are close to each other. l- n, l, j, m And the new lines are close to each other. can take the following values:
jB =- B gm j , E = B gm j , ( j =0, 1,…, J).= 1, 2, 3,…; l= 0, 1, 2,…, jB =- B gm j , E = B gm j , ( j =0, 1,…, J).- 1; And the new lines are close to each other. = l + l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. chi | l - l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. |; l It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign.= ±1/2;
-j? And the new lines are close to each other. m
? j. The value of the orbital moment l is indicated in spectroscopy by the letters s, p, d, f, etc. jB =- B gm j , E = B gm j , ( j =0, 1,…, J). Put the quantum number in front of the letter. And the new lines are close to each other. Enter the number at the bottom right j. Therefore, for example, rhubarb (term) with

= 3, l = 1, It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign.= 3/2 means 3 j. R l 3/2.
Figure 21 shows a diagram of the ranks of a hydrogen-like atom with a multiplet structure. Lines 5890? and 5896? assert And the new lines are close to each other. This is a doublet for sodium: yellow lines D2 and D1. The position of the electron near the central force field was designated by three quantum numbers: And the new lines are close to each other. 2 And the new lines are close to each other.-therm far inserted into 2 It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign.-terms, as they may be in water-like atoms ( j.-Virozhenya accepted). The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin., The position of the electron near the central force field was designated by three quantum numbers: And the new lines are close to each other. To every person from the glances of equals
E nl
lay down (2 + 1) stations that vary in number, then the orientation of the constant moment J is in space. . Only when an external field is applied, rivals who are angry can separate. + 1) stations that vary in number For the absence of such a field we can (2 .+ 1)-fold virogen. Bohr magneton So term 2 + 1) stations that vary in number 1/2 May Virgo 2: two legs that are divided by the orientation of the spin. . Term 2 + 1) stations that vary in numberі . 3/2 May four times the gyration is consistent with the orientation of the moment + 1) stations that vary in number= ±1/2, ±3/2.
EFFECT TO ZEIMAN. + 1) stations that vary in numberі P. Zeeman, including the spectrum of vibration of sodium vapor placed in an external magnetic field, revealed the splitting of spectral lines into a number of components. Over the years, on the basis of quantum mechanical phenomena, this phenomenon was explained by the splitting of the energy levels of the atom in the magnetic field. Electrons in an atom can be found only in discrete units, and when changing these, there is a change in or removal of the light quantum. = Electrons in an atom can be found only in discrete units, and when changing these, there is a change in or removal of the light quantum. + 1) stations that vary in number + The energy of the atomic energy lies in the total orbital moment, which is characterized by the orbital quantum number . L The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin., and the total spin of the electrons, which is characterized by the spin quantum number + 1) stations that vary in numberі .. The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin.. This kind of splitting is called the fine structure of ribs. Signs of fine structure appear to be split and spectral lines. + 1) stations that vary in number = 1 , . For example, + 1) stations that vary in number = 0, . D The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin.-the sodium line indicates the transition from the level The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin.= Ѕ ( The following shows that atoms with one optical electron can form doublets due to two orientations of the electron spin. =+ 1) stations that vary in number + .; .= ½ for equal s This kind of splitting is called the fine structure of ribs.= S.
First (Rivniv) - doublet, consistent with possible meanings And the new lines are close to each other.= 3/2 ta And the new lines are close to each other.= ±1/2), and the other does not have a fine structure.
Tom - the line consists of two very close lines with dovzhins hvil 5896?
and 5890?. The skin rhubarb of the multiplet is also deprived of morphology through the possibility of orientation of a constant mechanical moment in space (2+ 1) directly. j. The position of the electron near the central force field was designated by three quantum numbers: In the magnetic field, the degeneration is removed. The magnetic moment of an atom interacts with the field, and the energy of such interaction lies directly.і Therefore, the atom directly induces different additional energy in the magnetic field, and the Zeeman splitting of the level into (2) occurs+ 1) pіdrіvnіv.
Separate= normal (simple) Zeeman effect when the skin line is split into three components and abnormal (folded) when the skin line is split into more than three components. = To understand the hidden laws of the Zeeman effect, let’s look at the simplest atom – the water atom.
How to place a water atom near an external uniform magnetic field with induction IN, then for the ratio of mutual magnetic moment
From the external field, the atom adds an additional layer to the modules and mutual orientation j. U pm l energy UB The position of the electron near the central force field was designated by three quantum numbers: l-pmB
-pmBB,
de
pmB
- Projection of the magnetic moment of the electron onto the direct field. jB =- B gm j , E = B gm j , ( j =0, 1,…, J).і l Vrahovoyuchi scho l mB l= - ehm
/(2m) j.(l(magnetic quantum number It can also be explained that the magnetic moment of the valence electron due to the presence of the field can acquire two values, however, the one behind the modulus and the other behind the sign. (l= 0, ±1, ±2, …, ±l), can be rejected
Bohr magneton. The energy of an atom is equal to the magnetic field(l The first addition is the energy of the Coulomb interaction between an electron and a proton. From the remaining formula it follows that the absence of the magnetic field (B = 0) energy level is determined only by the first addition.(l When? The energy of an atom is equal to the magnetic field in the magnetic field

splits into five sub-trees, the stan - into three.
According to the rules for the transition, it is possible to proceed only by placing a baby.
As a matter of fact, the spectrum appears to have a triplet (normal Zeeman effect).
The normal Zeeman effect is avoided because the output lines do not have a fine structure (singlets).
If the output levels have a fine structure, the spectra appear to have more components and the anomalous Zeeman effect is avoided.
MECHANICAL AND MAGNETIC TORQUES OF THE ELECTRON
Orbital magnetic moment of an electron
Skin struma apparently generates a magnetic field.
Therefore, an electron whose orbital mechanical moment rises from zero is responsible for its magnetic moment.
From classical manifestations the moment of violence appears to the roc
de – fluidity, – radius of curvature of the trajectory.
The magnetic moment of a closed stream with a plane creates a magnetic moment
- One by one the normal to the area, i - the charge and mass of the electron.
Having corrected (3.1) and (3.2), we cancel
Magnetic moment coupled with mechanical moment multiplier
which is called magnetomechanical (gyromagnetic) conditions for electrons.
For the projection of moments we have the same connection
The transition to quantum mechanics involves the replacement of numerical equations with operator equations
Formulas (3.5) and (3.6) are valid not only for an electron in an atom, but also for any charged particles that generate mechanical torque.
Vlasne value of the operator is older
de - magnetic quantum number (div. Section 2.1)
This is called the Bohr magneton
Units of SI won become J/T.
This way you can also calculate the main value of the magnetic moment
de – orbital quantum number.
Vikorist post often
de.
The minus sign is omitted. Power mechanical and magnetic moments of the electron (spin) Stern and Gerlach (b. 1922) from spacious quantization.
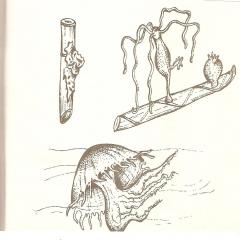
In these studies, beams of neutral atoms were passed through the region, creating a non-uniform magnetic field (Fig. 3.1).
In such a field, a part with a magnetic moment gains energy and exerts a force on it.
How can you split the bundle into separate components?
In the first experiments, beams of atoms were monitored. The beam was passed along the axis, preventing splitting along the axis..
The main warehouse strength is ancient
If the atoms are not awakened and are located on the lower level, then in the state (), then the beam is not liable to split, leaving the orbital magnetic moment of such atoms at zero.
For awakened atoms () the beam of bi-splits into unpaired particles, the number of components is equal to the number of possible values of the magnetic quantum number (). In reality, the beam was prevented from splitting into two components. This means that the magnetic moment that causes splitting has two projections on the direction of the magnetic field, and the corresponding quantum number takes on two values. The results of the experiment prompted the Dutch physicists Uhlenbeck and Goudsmit (1925) to propose a hypothesis about the presence of mechanical and associated magnetic moments in the electron Following the analogy with the orbital number, we introduce the quantum number, which characterizes the power mechanical moment of the electron.
The splitting is significant due to the complexity.
Otje,
The quantum number is called the spin quantum number, and it characterizes the power and spin moment of the spin of the hand (or simply “spin”).
Let us return to the experiments of Stern and Gerlach.
Knowing the magnitude of the splitting (by magnitude), it is possible to determine the magnitude of the projection of the spin magnetic moment onto the direct magnetic field.
Vaughn becomes one Bohr magneton.
We remove connections between and:
Magnitude
is called a spin magnetomechanical relationship and is twice as large as an orbital magnetomechanical relationship.
This is the connection between spin magnetic and mechanical moments: